Hydrogen cracking, also known as hydrogen-induced cracking or HIC, is a common concern in various industries, particularly in the field of metalworking, welding, and construction. This phenomenon occurs when hydrogen atoms infiltrate the microstructure of a metal, causing it to become brittle and prone to cracking. Understanding the common causes and effective cures for hydrogen cracking is crucial for engineers, welders, and professionals in industries where the integrity of metal structures is paramount. In this article, we will discuss the key factors behind hydrogen cracking and explore strategies to prevent and mitigate this issue.
What is Hydrogen Cracking
Hydrogen cracking, also referred to as cold cracking or delayed cracking, is a particular type of fissure that primarily occurs in weldable ferritic steels. Typically, these cracks occurs shortly after welding, either immediately or within a 48-hour window. The underlying mechanism begins with solitary hydrogen atoms diffusing through the metal.
At elevated temperatures, the increased solubility of hydrogen allows it to diffuse into the metal, although hydrogen diffusion can also occur at lower temperatures aided by a concentration gradient. As these hydrogen atoms reassemble within tiny voids within the metal matrix to form hydrogen molecules, they exert pressure from within these voids. This pressure can elevate to levels where the metal’s ductility and tensile strength decrease, ultimately leading to the formation of cracks (referred to as hydrogen-induced cracking or HIC). Materials like high-strength and low-alloy steels, nickel, and titanium alloys are particularly susceptible to this phenomenon, as is austempered iron.
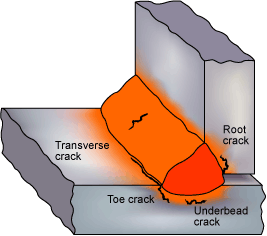
Fig. 1 Hydrogen cracks originating in the HAZ and weld metal.
Hydrogen cracks can be usually have the following characteristics :
- In C-Mn steels, these cracks usually initiate in the heat-affected zone (HAZ) but can also extend into the weld metal.
- Cracks might also occur within the weld bead, often running perpendicular to the welding direction at an angle of 45 degrees relative to the weld surface. They typically have a straight yet jagged path.
- In low-alloy steels, the cracks can be transverse to the weld, running perpendicular to the weld surface. These cracks tend to be planar in nature (referred to as planar defects). When the weld is fractured open, the crack surfaces generally lack oxidation, even if they are exposed on the surface. This lack of oxidation indicates that they formed when the weld was at or near ambient temperature, with a possible faint blue tinge from preheating or welding heat.
- Cracks originating in the HAZ are typically associated with the coarser grain regions. These cracks can be intergranular, transgranular, or a combination of both. Intergranular cracks are more likely to occur in the harder HAZ structures found in low-alloy and high-carbon steels, while transgranular cracking is more commonly observed in C-Mn steel structures.
- In fillet welding, cracks in the HAZ are often linked to the weld root and run parallel to the weld, whereas in butt welds, HAZ cracks are usually oriented parallel to the weld bead.

Fig. 2 Crack along the coarse grain structure in the HAZ
Common Causes of Hydrogen Cracking
1. Hydrogen Sources
One of the primary causes of hydrogen cracking is the presence of hydrogen sources during the fabrication or welding process. These sources can include moisture, rust, or contaminants on the metal surface, which release hydrogen when exposed to high temperatures. In some cases, the base material itself may contain dissolved hydrogen, especially in steels and other alloys.
The primary origin of hydrogen arises from the moisture present within the flux materials, encompassing the coating on MMA electrodes, the flux utilized in cored wires, and the flux employed in submerged arc welding. The quantity of hydrogen generated is subject to the type of electrode used.
Typically, basic electrodes tend to produce a lower amount of hydrogen in comparison to rutile and cellulosic electrodes. It is essential to acknowledge that there are additional notable origins of hydrogen, such as those originating from the material itself, where the steel may retain a considerable level of hydrogen due to its processing or service history, as well as moisture absorbed from the surrounding atmosphere. Furthermore, hydrogen may also emanate from the material’s surface or the consumable materials.
The sources of hydrogen encompass:
- Oil, grease, and dirt
- Rust
- Paint and coatings
2. Material Composition
When possible, choose materials that are less susceptible to hydrogen cracking. Understanding the properties and limitations of different alloys and metals is crucial in mitigating the risk of hydrogen-induced cracking.
The composition of the base metal significantly shapes both hardenability and the potential for developing a brittle, hardened structure in the Heat-Affected Zone (HAZ) when rapid cooling occurs. Typically, a material’s hardenability is quantified by its carbon content or, when considering additional elements, its carbon equivalent (CE) value.
Below we have provided a carbon equivalent calculator for your ease want to read about carbon equivalent read our article
The higher the CE value, the greater the risk of hydrogen cracking. Generally, steels with a CE value of <0.4 are not susceptible to HAZ hydrogen cracking, as long as low hydrogen welding consumables or processes are used.
3. Stresses acting on the weld
Residual stress, which results from the difference between the cooling rates of the weld and base material, is another factor in hydrogen cracking. This stress can lead to cracking in the heat-affected zone. Additionally, improper welding sequences can increase the residual stress, making the structure more susceptible to cracking.
Certain areas, such as the toe and root of the weld, are more susceptible to the initiation of cracks. The stresses that arise during the contraction of the welded joint are significantly affected by factors like external restraint, material thickness, joint geometry, and fit-up. In particular, inadequate fit-up, such as an excessive root gap in fillet welds, significantly elevates the likelihood of crack formation. Additionally, the level of restraint imposed on a joint tends to rise as welding continues, primarily due to the growing stiffness of the assembly.
4. Heat input
The heat input to the material from the welding process, together with the material thickness and preheat temperature, will determine the thermal cycle and the resulting microstructure and hardness of both the HAZ and the weld metal.
Increasing the heat input will reduce the hardness level, and therefore reduce the risk of HAZ cracking. However, as the diffusion distance for the escape of hydrogen from a weld bead increases with increasing heat input, the risk of weld metal cracking is increased.
Heat input Calculator is provided below if want to read about heat input visit our article
Cures and Prevention Strategies for Hydrogen Cracking
1. Preheating and Post-Weld Heat Treatment
Preheating and post-weld heat treatment are essential methods for reducing the risk of hydrogen-induced cracking. Preheating serves to eliminate any residual moisture within the base material that could otherwise contribute to the presence of hydrogen. Additionally, it helps to decelerate the cooling rate following the completion of the weld, providing extra time for the hydrogen to disperse from the weld. Likewise, raising the temperature of the weldments after welding can facilitate the more efficient diffusion of hydrogen.
2. Select the Low Hydrogen Filler/Electrodes
Metal-cored and flux-cored wires and stick electrodes are typically available in low-hydrogen varieties. In the American Welding Society (AWS) classification, there is an optional designation for diffusible hydrogen. Filler metals with H4 or H8 designators have undergone tests to demonstrate that they deposit weld metal containing low levels of diffusible hydrogen.
Stick electrodes can have an additional designation “R,” indicating that the electrode is resistant to absorbing moisture. This resistance to moisture absorption is crucial in maintaining low hydrogen levels during welding.
Solid wires also offer low hydrogen levels since they do not have filler material, making them less susceptible to moisture pickup and hydrogen-induced cracking. This is because the absence of filler material reduces the risk of introducing additional hydrogen into the welding process, which can lead to weld defects and cracks.
3. Minimize Joint Stress
Excessively constrained joints may exacerbate hydrogen-induced cracking, as they are inherently pre-stressed, which can amplify the additional stresses introduced by hydrogen during welding, ultimately resulting in the formation of cracks. Whenever feasible, aim to design joints with reduced stress levels to mitigate this issue.
Wrapping Up
Hydrogen cracking poses a significant challenge in industries where the integrity and safety of metal structures are paramount. Understanding the common causes of hydrogen cracking and implementing appropriate prevention and mitigation strategies is crucial. By addressing issues such as hydrogen sources, welding processes, preheat and interpass temperatures, residual stress, and material composition, professionals can minimize the risk of hydrogen-induced cracking and ensure the reliability of their welded structures. Careful attention to welding techniques and quality control measures can go a long way in preventing hydrogen cracking and preserving the structural integrity of the final product.
Related Article : What Is Hot Cracking (Solidification Cracking)?
